In this podcast Oskar Hoff, Head of Medicinal Chemistry, talks to Amir Amanzadi, Head of Comp. Chemistry, about degraders in general, and how they work compared to inhibitors.
Insight One
- The beginning of Celeris Therapeutics
- What role does AI play?
- What are degraders?
- Why degraders, and what’s the impact?
- How does a job as Machine Learning Researcher look like?
- How does a job as Computational Chemist look like?
- What’s Celeris One, and how does it work?
Transcript
Amir: Welcome to the third episode of insight one from Celeris Therapeutics, a deep tech company in the intersection of machine learning and life sciences. My name is Amir, and I’m the head of computational chemistry at Celeris Therapeutics. And I’ll guide you through this episode. This series contains 7 episodes, and called inside one, it shows you the insights of Celeris Therapeutics. It is my pleasure to have Dr. Oskar Hoff, our head of medicinal chemistry, as salutes therapeutics, as my guest. Oscar is an experienced organic chemist and recently earned his Ph.D. from the distinct university of Oxford. We will discuss the various chemical aspects of degraders in-depth and how we tackle the challenges we face and this unchartered territory.
Oskar: Hi Amir, thanks for having me.
Amir: So, Oscar, let’s start from the basics. We hear these small molecules, drugs also known as pharmaceutics. And we also hear about biological drugs a lot. So could you perhaps explain their differences and sort of clarify what the focus of Celeris Therapeutics is?
Oskar: Yeah, of course. So let’s start with the difference between proteins and small molecules. I’ll start with proteins. Usually, they act as enzymes. It’s a very hard simplification to view proteins just as enzymes, but for the time being and for this discussion today, it’s I think it’s going to be sufficient to just view the enzyme part of proteins.
There are other tasks that they do, but for now, that’s enough. So these enzymes catalyze biochemical reactions in our bodies. That sounds quite complicated, but basically, what they’re doing, they’re enabling processes in any living organism, and those are processes like moving our muscles that digest our food or building up body tissues, which are, of course, vital for us. Structurally speaking proteins, consist of amino acids joined together by two long chains. And these chains then fold up into various 3d structures. And these structures are much, much larger than a small molecule. And then small molecules, on the other hand, can be very small.
As the name suggests, they are very small. A small molecule, for example, is ethanol, which many of us consume when we drink wine or beer. A famous example of a small molecule drug is salicylic acid, and this is better known as aspirin by its trade name. Here at Celeris Therapeutics, our expertise lies in the development of small molecules degraders.
So those are small molecules that cause protein degradation.
Amir: Interesting. So as I understood it, it was lots of words in there, but essentially we have these biological entities which are body-consistent do what’s a functionality. And also, we have these small molecules that we utilize to cure diseases and etc.
So for me, is that exactly how these small molecules interact with our body?
Oskar: Okay. So small molecules can interact with proteins in various ways. So now we’re talking about a small molecule that is a drug and a protein that is native. So it’s part of our body, and if they interact and we can talk about a ligand-protein interaction.
So if a small molecule acts as a ligand towards a protein, it interacts. And it binds to that protein. The majority of consisting, small molecule drugs are so-called inhibitors. And those inhibitors, ideally they bind specifically to one enzyme or to a specific enzyme. They do that in a certain part of the protein, and we call this certain part an active site.
And these active sites are usually used by the enzyme to catalyze those biological processes that we talked about before, but the inhibitor, but binding this active site, blocks the function of the enzyme. So essentially, if we say this enzyme is now not part of our body, but it is part of our body, but it’s part of cancer, for example, and we can block this enzyme. Then cancer dies, and the patient recovers from the disease.
Amir: So that’s why the analogy of key and lock is always used when we talk about drug discovery. So essentially, these ligands are the key, and they bind to the active side and sort of change their functionality or either block or inhibit the functionality.
So, okay. In that case, how are degraders different exactly?
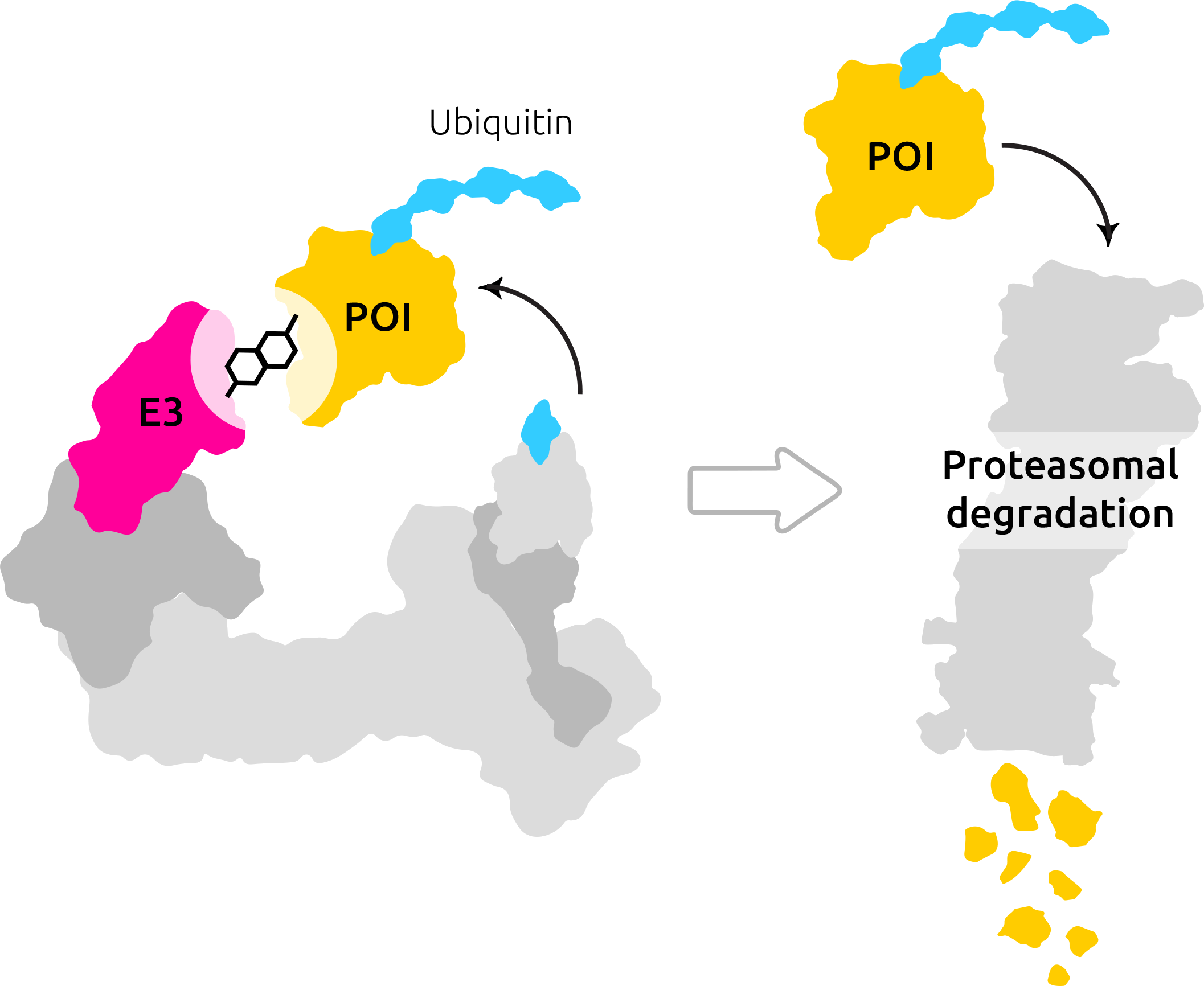
Oskar: So your analogy with key and lock is very good. And that’s often used. So degraders are completely different in terms of that, they don’t try to block the lock by competing with other keys to fit in there, but they will completely remove the whole lock.
So what they’re doing. They use mechanisms in our body that will ensure that our proteins are degraded, and then the lock has gone.
Amir: So could you elaborate on that because you have? I understand many other competing inhibitors want to inhibit the proteins, but how can we really remove a protein?
Oskar: Yeah. So there are multiple methods that our bodies use to recycle proteins. The mechanism that we’re interested in is the proteasome. So sort of proteasome, as I said, is a naturally occurring mechanism to recycle proteins. It occurs in all eukaryotic cells. And the way this happens is that when our cells think that a protein has outlived its purpose, it gets labeled by a so-called ubiquitin tech.
Proteasome recognizes this ubiquitination or ubiquitin tech and thus digests the attack protein and the MEU assets, and the building blocks can then be recycled within the cell.
Amir: Interesting. So I understand our body has essentially this deleting function of, you know, removing the on bonded proteins and like any biological entities, but how we can really use this in our case.
Oskar: So the ubiquitination is catalyzed by another enzyme. This enzyme is called E3 ligases. Generally speaking, enzymes are named after the reaction they enable, and ligase causes ligation, and that’s the binding of another molecule in another. In our case, this other molecule is the ubiquitin tech we already talked about, and this is catalyzed by the E3 ligase.
And our degraders are designed in such a manner that they bring the E3 ligase with the ubiquitin tech in close proximity to our protein of interest, causing ubiquitination of the protein of interest and subsequent degradation in the proteasome.
Amir: So I have heard of a person of interest but never heard of the protein of interest.
Could you little bit elaborate on that? Exactly.
Oskar: Yeah. So that protein of interest is basically the target that we select within the biological system that we are approaching, like our bodies when we treat cancer, for example. So you can also call it a target, and that’s the target we select for the degradation.
So if we are again with cancer, if you’re looking to treat cancer, we choose a protein that is unique and essential to this type of cancer. And then, we choose it as our target or protein of interest.
Amir: Okay. Really nice. So if I want to put this in sort of perspective at these degraders, bring the protein of interest close to the E3 ligase, right?
And the proteins get ubiquitinated. And these approaches, you know, and recognize the ubiquitination on these proteins of interest and break to proteins in the building blocks. Right. So what happens to the prototype? Would it remain there and do the process again?
Oskar: The process can actually remain there.
So after ubiquitination took place, um, it can be recycled. It can be reused.
Amir: Okay. So these small molecule degraders look like, what is the composition chemically speaking? What are the differences between the small molecules?
Oskar: We talked about small molecules just before, and, uh, degraders that we are talking about are also small molecules, just like aspirin.
However, they’re usually a bit more complex than small molecules, like ethanol or aspirin, because they have to fulfill multiple requirements. The first requirement is that they have to bind to the E3 ligase, and that part of the PROTAC is going to be called a binder. Secondly, they have to interact with the protein of interest, and we like to call this unit warheads.
And then, because we want to bring the E3 ligase and the protein of interest close together. We link the two parts. So we linked the binder and the warheads with the linker.
Amir: Okay. Understood. So we have essentially three parts, warhead linker, and binder. Okay. So does this linker have any other roles instead of, you know, just connecting warhead binder?
Oskar: Absolutely. Yeah. So a linker is not just a piece of molecular string that keeps the binder, the word. There are multiple reasons why it is actually important what kind of link do we use. They’re very simple things like solubility and permeability of the cell, but specifically for this interaction to take place, the length and flexibility of the linker are crucial.
So those proteins, the E3 ligase, is a protein, and the protein of interest, they also interact with each other. And that’s why the length and the flexibility are important because the event when the three come together, so the E3 ligase, the small molecule degrader, and protein of interest, all three parts are in a complex together.
It is what we’d call turnery complex formation, and this is paramount for ubiquitination and then protein degradation afterward. And moreover, the linker is also important for the selectivity of this interaction. So let’s say we’re still looking for treatment for this cancer that we talked about. And so, we selected a protein that is specific to cancer, but the human body of the patient also has a similar protein.
It’s not identical to the protein of the cancer, but it’s similar enough, and we really don’t want to harm the patient because obviously, that’s not good. So we are looking to make a degrader, the selective for the cancerous protein, but not the selective protein of cancer over the protein of the patient. And that’s when we talk about targeted protein degradation, and the linker plays a vital role in that.
Amir: Lots of moving parts here. And I guess I get the understanding, like what is the difference between degraders and inhibitors? So just for the audience out there, to my understanding, inhibitors blocked enzymes under functionality by binding to the active side, but degraders kind of remove these proteins completely, sort of the victim.
I understand these are going to compound the different and, you know, chemically different, but conceptually how they are really different. So, you know, if we have inhibitors, right, what do degraders put on the table. Why should we work on this?
Oskar: So it’s a very good question. First of all, inhibitors have been around for much longer.
And most of the drugs that we know so far, small molecule drugs, are inhibitors, and targeted protein degradation is quite new. So it’s an emerging field. Thus, there are more opportunities for us, but there are also more unanswered questions for us to tackle when it comes to the target protein, degradation. And as discussed earlier, once at a degrader molecule causes degradation, and the protein of interest is labeled and is going to be disposed into the proteasome.
The degrader can interact with the next protein of interest. So this is like a catalytic mode of action. Whereas the inhibitor it’s stuck in the active site, and it’s stuck there to do its job at best, but the degrader can label one protein of interest after the other. I conceptually very important difference to inhibitors because that allows that catalytic mode allows the degraders to act at lower concentrations.
So basically, you have to take smaller pills. You don’t have to take as much into your body, which is a problem for toxicity to get the same effect as an inhibitor. And more importantly, the greater warheads don’t have to bind the protein of interest as tightly as an inhibitor needs to because it doesn’t have to compete with the natural substrate of the enzyme.
So you can also use interactions, or you can make use of interactions that are not as. Because sometimes quite tricky to develop those strong interactions. Another point is that inhibitors have to block the active site, which we talked about before, but a degrader just has to interact with this, with the protein of interest selectively over other proteins.
But it doesn’t matter where it interacts. You have a whole surface of the protein to create interaction with, which gives us many more chances. And actually, I can’t emphasize those points enough. So we need an active site for an inhibitor, but for a degrader, we don’t need an active site.
So we can actually tackle targets, proteins interests, which don’t have active sites. So basically are undruggable so far, and the diseases they were causing were incurable, and those targets aren’t exactly diseases, um, and targets that we are interested in.
Amir: Fantastic. So to me, there were two major takeaways. First, these degraders are catalytic.
So after they have done the degradation, they stay in our body, and we can take and do this over and over again. So it’s kind of good for drug resistance in most cases. Also, because we don’t need that active site, you know, binding then is, let’s say, cancer or HIV. They mutate the binding site doesn’t change.
So we can still use the same degraders in our body and, you know, complete the degradation. So, but it’s not that easy, is it right? So exactly what, what are we trying to do in Celeris Therapeutic to get there?
Oskar: Here at Celeris Therapeutics. We have a very diverse team of scientists, and we’re looking to develop a heterobifunctional degrader. We have a very strong team of machine learning, engineers, and programmers. They are working on an in silico solution to design, degrade, and predict ternary complexes between the protein of interest, the degrader, and the E3 Ligases. I don’t want to talk about this in too much detail here because I’m sure the following episodes will explain this much better than I can.
And in addition to this in silico machine learning studies, we have an impressive team of computational chemists, led by you, Amir. And then, in the wet lab, we have a biology and a medicinal chemistry team. And together, we want to develop a pipeline where we can actually accelerate this drug developing process for degraders, as we know it currently.
Amir: Fascinating topics. And I’m eager to see where this interdisciplinary adventure will take us. It is certainly a great vision for the upcoming years and clear paths to get there. Thank you very much, Oscar, for that delightful discussion. In the next episode, we’ll be talking about the impact of degraders and thank you to our listeners. for listening to this lovely episode and wish you all luck, have a good day.