If you have read the “The Future Is Faster Than You Think” by by Peter H. Diamandis and Steven Kotler you will see that they defined the nine main “causes” of our decline, the nine horsemen of an internal apocalypse. The question I wanted to answer is:
Can we frame these problems/horsemen computationally in terms of machine learning and then try to solve them?
PS: We are not pivoting, just judging by previous announcements by some of the competitors it seems we were on the right track all along 😉
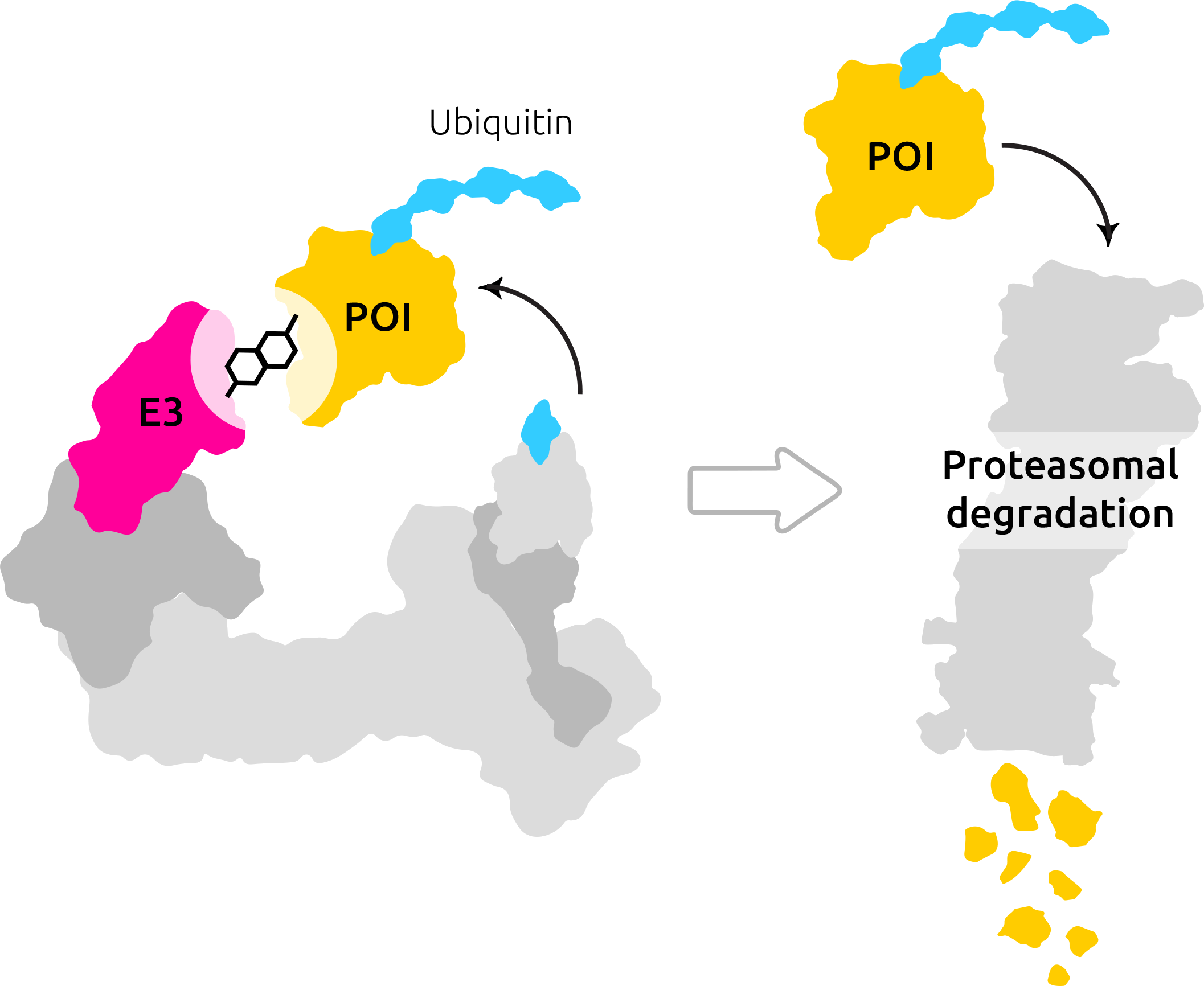
Before we start, we should define what suffices as “solving it computationally with AI”. AI is enabling technology, not an end to end solution. There are parts of the process in the real word (like the drug discovery process) that can be automated with AI. For example what we are doing at Celeris. But realistically, current AI is (mostly) not able to build end to end solutions for most of the complex real world problems out there. This is all important to say because tackling longevity with AI does not mean building an end to end solution but rather automating the parts of existing solutions and making it better.
So what exactly is killing us?
- Genomic instability: DNA doesn’t always replicate according to plan. Typically, these errors in gene expression get caught and corrected, but not always. Over time, these misfires build up, causing our body to wear down — meaning genetic instability leads to genetic damage leads to a limit on lifespan. Think of it as a broken copy machine, except, instead of producing unreadable pages, our broken genetic copier produces diseases like cancer, muscular dystrophy and ALS. Data? HumCFS: a database of fragile sites in human chromosomes. What could you do with it? Identify other fragile chromosomes sites AND link them to other diseases based on atom/chemical level properties.
- Telomere Attrition: At the heart of a cell, DNA is packed into threadlike structures called chromosomes. Chromosomes are capped by telomeres which act like barriers — like bumpers on a car — designed to protect the core of the chromosome. But as DNA replicates, telomeres get shorter and at a critical level, the cell stops dividing, and we become more prone to disease and death. It has been shown that if one can increase or reduce the shrinkage of telomere on mice, longevity increases. Data? Telomerase Database What could you do with it? Telomerase can be used as a target in anti-cancer therapy. What this essentially means is one can find computationally (in-silico) inhibitors that hinder the shrinkage of telomerase. Doing this which we know has positive side effects for longevity.
- Epigenetic Alterations: Nature impacts nurture. Over the course of a lifetime, environmental toxins can change how our genes express, sometimes for the worst. Exposure to carcinogens in the environment can silence the gene that suppresses tumors for example. But also the other way around, we can silence the gene that is responsible for cancer. Data? dbEM and GenAge: A database of epigenetic modifiers curated from cancerous and normal genomes. What could you do with it? Epigenetic proteins (modifiers) are targets in cancer. With this database we get not only the protein but also genomic information pertaining to mutational status, copy number variation and expression level of epigenetic proteins in tumor samples. This enables explorationof other protein that were not classified as epigenetic, based upon their genomic information. How to utilise GenAge? There are tools and companies out there that allow for a complete measurement of one genes. ML models could be built that recommend actions based on certain gene structure.
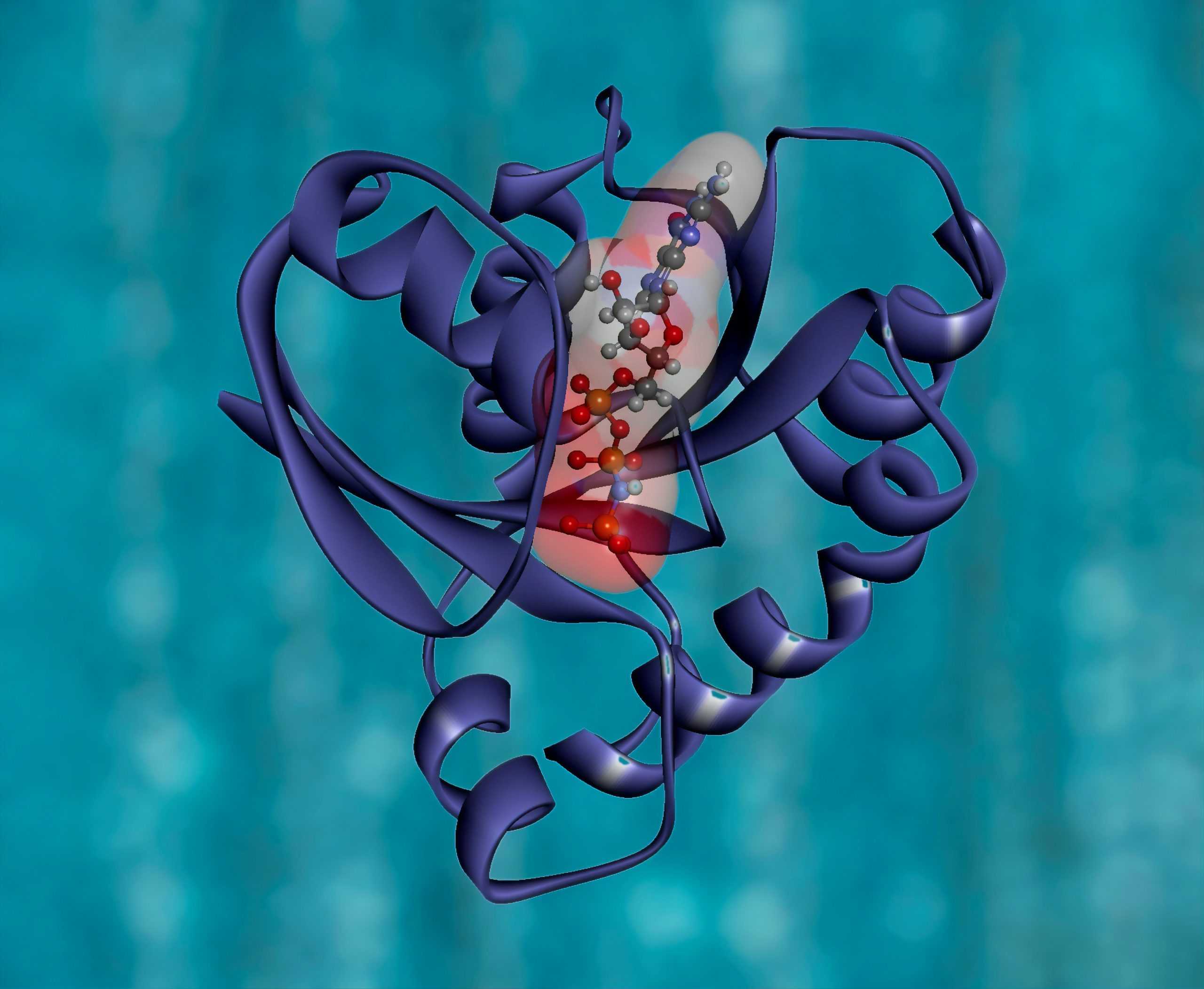
- Loss of Proteostasis: Inside a cell, proteins run the show. They transport materials, send signals, switch processes on and off, and provide structural support. But proteins become less effective over time, so the body recycles them. Unfortunately, as we age, we can lose this ability. The trash collector goes on strike and we suffer a toxic buildup of proteins that can, for example, lead to diseases such as Alzheimer’s. Data? AlphaFold: Protein Structure Database. What could you do with it? Proteins that become terminally misfolded, or are no longer functionally required, they must be degraded to avoid damaging effects of their continued presence, predicting these proteins for shapes that we do not know could be essential in targeting their degradation. Which is exactly what we have achieved with Celeris (using a different approach)
- Nutrient Sensing Goes Awry: The human body relies on over forty different nutrients to stay healthy. For everything to work perfectly, cells need to be able to recognize and process each of these. But this ability breaks down as we get older. For example, one reason people gain weight as they age is that our cells can no longer properly digest fat and carbohydrates. And one reason we die is that this impacts the insulin and IGF-1 pathway and can result in diabetes. Data? Nutrient Sensing Mechanisms and Pathways. What could you do with it? GenDR Database of Dietary Restriction-Related Genes. Not a direct ML approach but utilising advancements in wearables and mobile sensors to get a more granular and personalised data on nutrition intake based on your current metabolic state could have huge impact. One can easily imagine getting measuraments on nutrient sensing of different (cancer) cells in the body and then depending on that information adjusting the nutrient intake such that the desired effect on cells is achieved. For example death of cancer cells. Extending this to genes one can use the GenDR Database of Dietary Restriction-Related Genes
- Mitochondrial Dysfunction: Mitochondria are power plants. By converting oxygen and food into energy, they provide the basic fuel for our cells. But performance declines over time. The result is free radicals, a damaging form of oxygen that mangles DNA and proteins and leads to many of the chronic illnesses associated with aging. Data? MSeqDR Mitochondrial Genomic Tools and Databases What could you do with it? With this currated list of databses one could combine measurements of Oxygen consumption ratio (OCR), maximal oxygen consumption and mitochondrial reserve capacity and their respective proteins. Beeing especially interested in proteins that are targets in certain diseases, one could then have clear indicator with wearables or other measurement devices wether some disease is going to be triggered.
- Cellular Senescence: As cells undergo stress, they occasionally become “senescent”, both losing their ability to divide and becoming resistant to death. These “zombie cells” can’t be removed from the body. They build up over time, infect neighboring cells, and ultimately create a zombie apocalypse of inflammatory debilitation. Data? CellAge Database of Cell Senescence Genes What could you do with it? Based on our personal gene makeup we could have model that recommend altering of genes associated with cellular senescence. Instead of altering one can do further analysis of what action is needed for the genes involved in the sensescence.
- Stem Cell Exhaustion: As we age, our supply of stem cells plummets, in certain cases by a ten thousand fold decline. Worse, the ones we do manage to hang on to become far less active. This means that the body’s internal tissue and organ repair system loses its ability to do its job. Now we already know that stem cells help and there are therapies out there for them. But how can AI make this even better? Data? Stemformatics: visualize and download curated stem cell data What could you do with it? A curated list of potenital AI applications in stem cell research can be found here. One crucial application in designing stem cell therapies for central nervous system (CNS) diseases is differentiation of neural stem cells (NSCs) into neurons. Differentiation is complex and not yet clearly established, especially at the early stage. Novel nature publication shows that deep learning could extract minutiae from large-scale datasets, and present a deep neural network model for predictable reliable identification of NSCs fate.
- Altered Inter cellular Communication: For the body to function properly, cells need to communicate. This happens constantly, with messages flowing through our bloodstream, immune system and endocrine system. Over time, signals get crossed. Some cells become unresponsive, others become inflammation-producing zombie cells. This inflammation blocks further communication. Once this happens, messages can’t get through and the immune system can’t find pathogens. Data? PanglaoDB: is a database for the scientific community interested in exploration of single cell RNA sequencing experiments from mouse and human. What could you do with it? Developing a ML tool that can quantitatively infer and analyze intercellular communication would be useful because increases and decreses in signal between cells could be indicative of changes in the organism and other action should be taken. Novel nature publication develops such tool. CellChats.
Honorable Mention: DrugAge Database of Anti-Ageing Drugs contains an extensive compilation of drugs, compounds and supplements (including natural products and nutraceuticals) with anti-ageing properties that extend longevity in model organisms. Now imagine building a model that intakes the atomic represenation of these compounds and tries to target some proteins in the human cells. More specifically proteins that are disease related. If one additionally uses our platform and DEGRADES the target before binding the drug, chances are one would discover and interesting thing or two 🙂
Notice the could not the can in the question. This is by no means extensive list of all of the AI things that can be applied. After all we got to pivot 😉